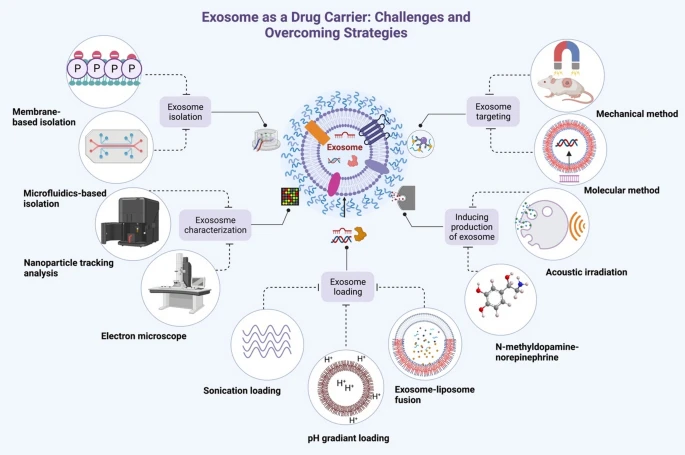
Challenges and Standardization
Despite their promise, exosome technologies face serious hurdles. Detection Sensitivity & Specificity:
Exosomal biomarkers are often present at low abundance and mixed with a background of vesicles
from normal cells. Current analytical methods may lack the sensitivity to reliably quantify key tumor
markers, leading to possible false negatives or false positives . In practice, distinguishing disease-
relevant exosomes from the normal EV pool is nontrivial. Methodological Variability: No single “gold
standard” exists for exosome isolation or analysis. Researchers use ultracentrifugation, size-exclusion,
precipitation kits or microfluidic chips, and these methods differ in yield and purity. As one review notes,
the heterogeneity of exosome sources and the lack of uniform protocols “pose a challenge for
standardization” . The International Society for Extracellular Vesicles (ISEV) has issued MISEV
guidelines and promotes tools like the EV-TRACK database to encourage rigorous, transparent reporting
. Nevertheless, concerted efforts are still needed to establish reproducible workflows and quality
controls. Only with standardized isolation and detection methods can exosome assays be validated and
approved for clinical use .
In summary, exosomes have come from obscurity to become a major focus of biomedical research.
These tiny vesicles carry rich information about their parent cells and have profound effects on recipient
cells. In oncology, the goal is to turn exosomes into a practical tool: a non-invasive “liquid biopsy” for
early cancer screening and a vehicle for targeted therapy. Early successes and ongoing clinical trials
underscore their potential, but hurdles remain. Overcoming challenges of sensitivity, specificity, and
standardization will be crucial. If achieved, exosome-based tests could revolutionize cancer detection by
offering a simpler, more accurate screening method than existing modalities .